Hey there, health enthusiasts! Today, we're diving deep into a topic that might make you think twice the next time you pop an antibiotic pill. Don't worry, we're not here to scare you – antibiotics are still life-saving marvels of modern medicine. But like that friend who always crashes on your couch and eats all your snacks, they might be overstaying their welcome in ways we're only just beginning to understand.
A groundbreaking study recently published in Science Advances has shed light on how antibiotics affect our gut health, specifically the protective mucus barrier in our colon. And let me tell you, it's not just about killing off those pesky bad bacteria anymore!
The Gut: Your Body's Unsung Hero
Before we dive into the nitty-gritty of this new research, let's take a moment to appreciate the wonder that is our gut. If you haven't already, check out our previous post on "The Gut Microbiome: Your Body's Hidden Ecosystem" for a primer on the bustling city of microbes living in your intestines.
Your gut is so much more than just a food processor. It's home to trillions of bacteria, forming a complex ecosystem that plays a crucial role in your overall health. These tiny tenants help you digest food, produce vitamins, and even communicate with your brain. Yes, you read that right – your gut and brain are in constant chatter, which we explored in our post on the gut-brain axis.
But how does your body keep all these bacteria in check? That's where our star of the show comes in – the mucus barrier.
The Mucus Barrier: Your Gut's Protective Moat
Now, imagine your gut as a medieval castle. The stones and mortar are your intestinal cells, but what's protecting them from invaders? That's right, the moat! In our gut, this moat is the mucus layer – a slippery, slimy barrier that keeps bacteria at bay and maintains peace in the kingdom.
This mucus layer isn't just any old slime. It's a sophisticated, two-layered structure:
The inner layer is firmly attached to the intestinal cells and is generally sterile. It's like the castle's inner sanctum, off-limits to all but the most trusted inhabitants.
The outer layer is looser and houses some of our friendly gut bacteria. Think of it as the castle town, where trusted allies can live and work.
This mucus barrier does more than just keep bacteria away from our intestinal cells. It also:
- Helps in nutrient absorption
- Acts as a lubricant for the passage of stool
- Contains antimicrobial compounds that help fight off harmful bacteria
- Provides a home for beneficial bacteria
In short, it's pretty darn important for our gut health!
The Antibiotic Revolution: A Double-Edged Sword
Now, let's talk about antibiotics. These medicines have been nothing short of revolutionary since their discovery in the early 20th century. They've saved countless lives by fighting off bacterial infections that were once deadly.
But as with many good things, there's a catch. We've known for a while that antibiotics don't just target the bad bacteria – they can also affect our beneficial gut bacteria. This disruption of our gut microbiome can lead to issues like antibiotic-associated diarrhea and, in some cases, more serious conditions like C. difficile infections.
However, this new study suggests that the effects of antibiotics on our gut go even further than we thought.
The Study: Unmasking the Hidden Effects of Antibiotics
So, what did this new study find? Well, hold onto your probiotics, because it's a doozy!
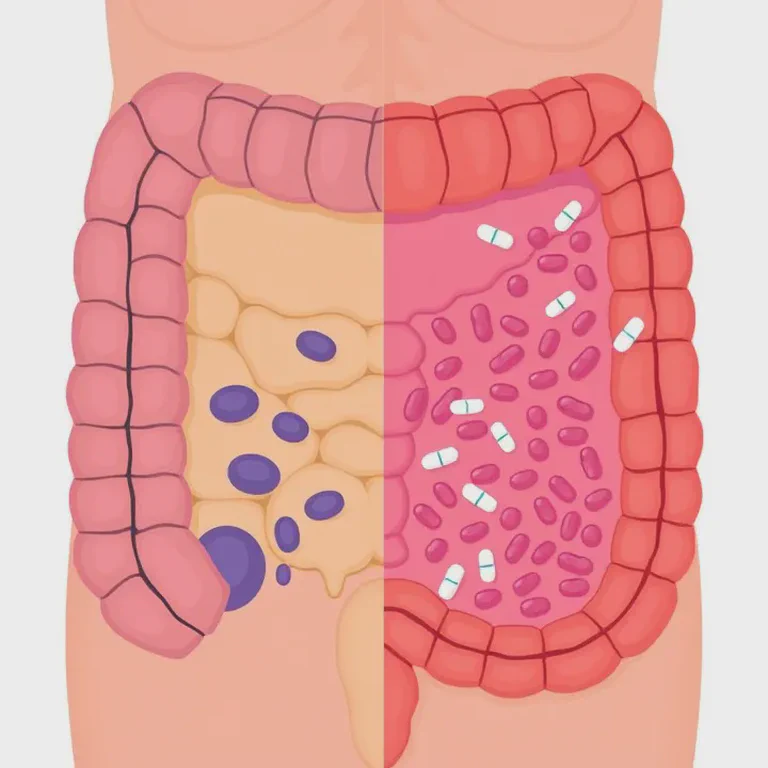
The researchers discovered that antibiotics don't just affect our gut bacteria (the microbiome) – they can directly damage the mucus barrier in our colon. It's like the antibiotics are not only killing the invaders but also draining the moat and weakening the castle walls!
Here's the breakdown of what they found:
1. Antibiotic Assault on the Mucus Barrier
The study looked at four different antibiotics: ampicillin, metronidazole, neomycin, and vancomycin. All of these, when given orally, led to a breakdown of the mucus barrier and allowed bacteria to get uncomfortably close to the intestinal wall. It's like the bacteria were getting VIP passes to a concert they definitely weren't invited to!
The researchers used some pretty cool techniques to visualize this. They used special staining methods and high-powered microscopes to actually see how close the bacteria were getting to the intestinal wall. In mice treated with antibiotics, the usually bacteria-free zone right next to the intestinal cells was being invaded.
2. It's Not Just About the Microbiome
Now, here's where it gets really interesting. The researchers were clever cookies and did something called a fecal microbiota transplant. Basically, they took the gut bacteria from antibiotic-treated mice and gave them to germ-free mice (mice with no gut bacteria).
Guess what? The germ-free mice didn't show the same mucus barrier breakdown. This suggests that the antibiotics were directly affecting the gut itself, not just changing the bacterial community. It's like the antibiotics were remodeling the castle without consulting the architect!
This is a big deal because it means that even if we could perfectly restore our gut bacteria after antibiotic treatment (which is challenging in itself), we might still be dealing with a damaged mucus barrier.
3. Stress in the Castle
The researchers found that antibiotics were causing stress in the cells lining the gut. Specifically, they were triggering something called endoplasmic reticulum (ER) stress. The ER is like the manufacturing plant of the cell, and when it's stressed, it can't produce proteins properly – including those needed for mucus production.
This ER stress led to a decrease in the production of mucin, the main component of mucus. It's like the castle suddenly running out of materials to maintain the moat!
4. Not All Antibiotics Are Created Equal
Interestingly, when the antibiotics were given systemically (like through an injection) instead of orally, only neomycin and vancomycin caused mucus barrier disruption. This suggests that different antibiotics might affect our gut in different ways depending on how they're administered.
This finding highlights the complexity of antibiotic effects on our body. It's not as simple as "antibiotics disrupt gut bacteria" – the story is much more nuanced.
5. The Gut-Brain Axis Twist
Remember how we talked about the gut-brain connection? Well, this study found that the vagus nerve, which connects your gut to your brain, might be involved in how antibiotics affect mucus secretion. It's like the brain is getting distress signals from the gut and doesn't know how to respond!
This adds another layer of complexity to the story and underscores how interconnected our body systems really are.
What Does This Mean for You?
Now, you might be thinking, "Great, another thing to worry about!" But don't panic! This research is helping us understand our bodies better, which is always a good thing. Here's what these findings might mean for you:
Antibiotic Awareness: This study reinforces the importance of using antibiotics judiciously. They're fantastic when we need them, but we should avoid unnecessary use. Next time your doctor prescribes antibiotics, don't be afraid to ask questions about whether they're absolutely necessary.
Viruses vs. Bacteria: It's crucial to remember that antibiotics are only effective against bacterial infections, not viral ones. If you have a viral infection like the common cold or flu, antibiotics won't help and could potentially harm your gut health unnecessarily. Always consult with your healthcare provider to determine if an infection is bacterial or viral before taking antibiotics.
Gut Health Focus: If you do need to take antibiotics, it might be worth paying extra attention to your gut health during and after treatment. This could involve dietary changes, probiotic supplements (under medical guidance), or other gut-supporting strategies.
New Treatment Possibilities: Understanding how antibiotics affect the mucus barrier could lead to new ways to protect our gut during antibiotic treatment. Researchers might develop methods to support mucus production or protect the barrier while we're taking antibiotics.
Personalized Medicine: The finding that different antibiotics affect the gut differently depending on how they're given could lead to more personalized antibiotic treatments in the future.
Broader Health Implications: A damaged mucus barrier might have effects beyond just gut health. It could potentially impact our immune system, our risk for certain diseases, and even our mental health (remember that gut-brain connection!).
Practical Steps: Nurturing Your Gut During Antibiotic Treatment
While we wait for science to potentially develop new ways to protect our mucus barrier during antibiotic treatment, there are steps you can take to support your gut health:
Probiotics: These friendly bacteria can help restore your gut microbiome. However, timing is crucial – some studies suggest taking probiotics a few hours before or after your antibiotic dose. Always consult with your healthcare provider before starting any new supplement.
Prebiotics: These are foods that feed your good gut bacteria. Think garlic, onions, leeks, asparagus, and bananas. Including these in your diet can help support your gut microbiome.
Fermented Foods: Foods like yogurt, kefir, sauerkraut, and kimchi contain beneficial bacteria that can support gut health. Just make sure to choose versions without added sugars.
Stay Hydrated: Drinking plenty of water can help support your digestive system and may help mitigate some side effects of antibiotics.
Nutrient-Rich Diet: Eating a diverse diet rich in fruits, vegetables, whole grains, and lean proteins can provide your body with the nutrients it needs to support gut health and overall well-being.
Stress Management: Remember that gut-brain connection? Managing stress through techniques like meditation, yoga, or deep breathing might help support your gut health too.
Follow Your Treatment Plan: While this research highlights some concerns about antibiotics, it's crucial to complete your prescribed course of antibiotics as directed by your healthcare provider. Stopping early can lead to antibiotic resistance, which is a serious global health concern.
The Future of Gut Health Research
This study opens up exciting new avenues for research. Some questions scientists might explore in the future include:
- Can we develop antibiotics that are less disruptive to the mucus barrier?
- Are there ways to protect the mucus barrier during antibiotic treatment?
- How does a damaged mucus barrier impact long-term health?
- Could mucus barrier disruption play a role in conditions like inflammatory bowel disease?
- How do other medications or environmental factors affect the mucus barrier?
As research in this area continues, we're likely to gain an even deeper understanding of our gut health and how to protect it.
A Word on Gut Health Beyond Antibiotics
While this post has focused on the effects of antibiotics on gut health, it's worth remembering that many factors influence our gut health. Diet, stress, sleep, exercise, and even our relationships can all impact our gut microbiome and overall digestive health.
Cultivating good gut health is a holistic process. It involves nourishing our bodies with a variety of foods, managing stress, getting adequate sleep, staying hydrated, and engaging in regular physical activity. It's about creating an environment where our gut can thrive, mucus barrier and all!
In Conclusion: Respecting the Complexity of Our Gut
This research on antibiotics and the mucus barrier serves as a reminder of just how complex and intricate our bodies are. Our gut is not just a simple tube that processes food – it's a sophisticated ecosystem that plays a crucial role in our overall health.
As we learn more about how various factors, including medications like antibiotics, affect our gut health, we become better equipped to take care of our bodies. This knowledge empowers us to make informed decisions about our health and to ask the right questions when we're seeking medical care.
Remember, antibiotics are still crucial medicines that save countless lives. This research doesn't mean we should avoid antibiotics when we truly need them. Instead, it highlights the importance of using them judiciously and taking steps to support our gut health when we do need to take them.
So, the next time you're prescribed antibiotics, think of your gut as that medieval castle. Yes, you might need to call in the antibiotic cavalry to fight off invaders, but make sure you're also reinforcing those walls and refilling that moat. Your gut will thank you!
Stay curious, stay healthy, and keep nurturing that amazing microscopic world inside you. Until next time, gut health enthusiasts!
Reference: Antibiotics damage the colonic mucus barrier in a microbiota-independent manner